The Invisible Risk
In Oral Health Care
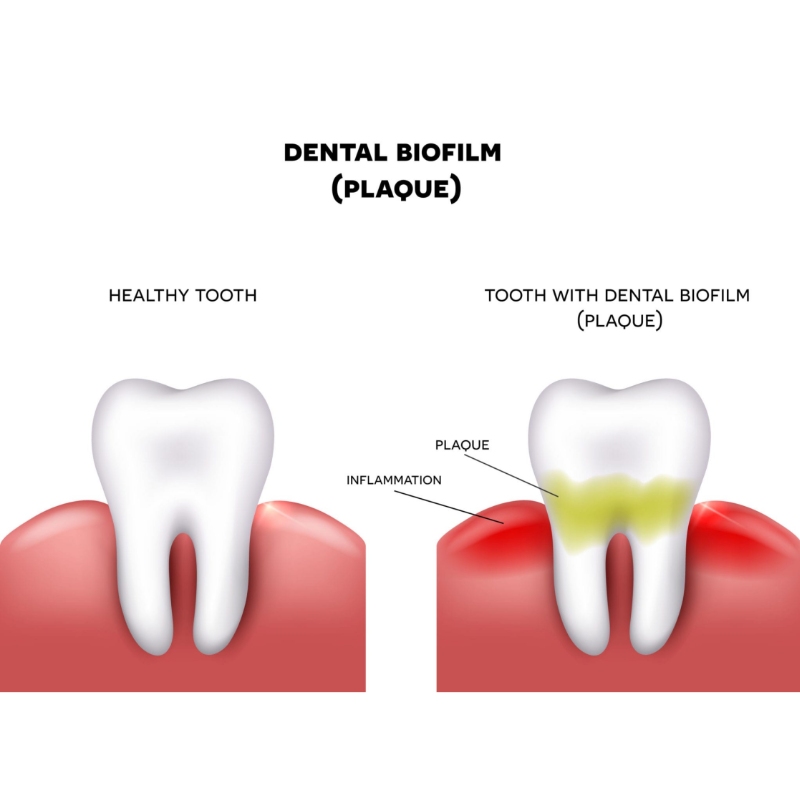
Dental plaque is the root cause of caries, periodontal disease, and implant failure, yet it remains largely invisible in daily hygiene routines. Plaque disclosing tablets have long been marginalized as mere accessories, but the clinical reality proves they are the missing link in preventative care.
See the difference professional manufacturing makes.
From "Novelty Item" to Clinical Necessity
For too long, plaque disclosing tablets have been misunderstood as cheap children's educational toys or one-time promotional giveaways. It is time to redefine their value proposition as professional oral management tools.
From "Toy" to Clinical Tool
Moving beyond "purple teeth fun" to precise, high-contrast biofilm identification that dentists rely on for periodontal management.
From "One-Time" to Routine
Transforming a single-use sample into a recurring weekly home-check habit that drives long-term consumer retention.
From "Messy" to Controlled
Replacing crumbling, staining cheap tablets with engineered formulas that offer rapid dissolution and easy rinsing without residue.
From "Generic" to Brand Asset
Elevating the tablet from a commodity to a branded compliance aid that enhances the perceived value of your total oral care kit.
Ready to elevate your product offering beyond the industry baseline? Explore our professional-grade formulations.
The Standardization Crisis
"Plaque Disclosing Tablet" is a term used loosely across the industry. Without unified manufacturing standards, products vary wildly in performance, leading to unreliable clinical outcomes.
Staining Intensity
Some tablets are too faint to show old plaque, while others use aggressive dyes that stain gums for hours.
Inconsistent educational value and consumer frustration.
Dissolution Instability
Cheap fillers cause tablets to become rock-hard or crumble into powder during transit.
Poor user experience and high breakage rates.
Ingredient Opacity
Many generic tablets lack traceable lists, blurring the line between food-grade dyes and industrial colorants.
Safety concerns and failure to pass import inspections.

Don't settle for "good enough." Demand a consistent, measurable standard.
The High Cost of Low-Price Sourcing
The market is flooded with ultra-cheap, generic disclosing tablets. For B2B buyers, the upfront savings often evaporate when faced with the hidden costs of non-compliance and reputational damage.

The Regulatory Void
- Missing FDA registration or CE technical files.
- Generic COAs that don't match specific batches.
- High risk of customs detention or recall.
Safety Roulette
- Unverified dye sources causing potential allergic reactions.
- Lack of microbiological testing reports.
- No traceability for raw materials.

Brand Liability
- Negative reviews due to staining or taste.
- One quality failure damages trust in your entire product line.
- Loss of professional (dentist) referrals.
The Disconnect Between
Clinical Goals & Consumer Reality
Dentists prescribe disclosing tablets for precise education. Consumers buy them for home care. But most products on the market fail to serve either group effectively because they aren't designed for the real usage scenario.
What Clinicians Want
Professional Precision Target Standard
Target Standard
-
Clear Differentiation
Sharp contrast between old (dangerous) and new plaque.
-
Controlled Residue
Stains only the biofilm, not the gums, lips, or sink.
-
User Friendly
Rapid dissolution and easy rinsing encourage compliance.
The Market Reality
Common Complaints Current Frustration
Current Frustration
-
Over-Staining
Whole mouth turns purple; stains difficult to remove from face/clothes.
-
Bad Taste & Texture
Chalky texture and chemical taste deter repeat usage.
-
Unclear Results
Blurry indication makes it hard for users to know where to brush.
Your brand needs to bridge this gap. We manufacture to the Ideal Standard.
Why Has This Category
Lacked Innovation?
The quality issues aren't accidental—they are structural. Most factories treat plaque disclosing tablets as "just another compressed powder," ignoring the oral care science required for a functional medical device.
Generic "Pressing" Mentality
Most suppliers are generalist candy or supplement factories. They apply candy-making logic to a diagnostic tool, resulting in unstable dissolution and poor staining control.
Lack of Clinical Insight
Without dental professionals on the R&D team, factories don't understand how the product is used in a clinical setting vs. a home bathroom.
Blind Copying
OEMs often duplicate formulas from expired patents without validation, inheriting old flaws rather than optimizing for modern standards.
Buyer Disengagement
Brands treat this as a low-priority SKU, accepting "off-the-shelf" quality instead of pushing for differentiation and performance.
We Changed the Formula
We stopped treating disclosing tablets as a commodity. By bringing oral care science back into the manufacturing process, we created a new standard for B2B supply.
The New Plaque Disclosing Standard
Before you source your next batch, use this checklist to evaluate if your product is meeting modern clinical and consumer expectations.
Purple Disclosure
Must differentiate new plaque (red) from old, high-risk biofilm (blue/purple) for actionable feedback.
Rapid Dissolution
Tablets must break down completely in under 30 seconds of chewing without gritty residue.
Traceable Ingredients
Food-grade dyes with clear origin documentation, ensuring safety for daily use by children.
Redefining Solutions via Manufacturing
True product reliability isn't just about the formula—it's about the process. We engineer stability into every tablet.
Controlled Compression
We use specialized low-force compression technology to ensure tablets are hard enough to ship but soft enough to dissolve instantly upon chewing.
Humidity Management
Production happens in strictly dehumidified environments (<40% RH) to prevent premature activation of effervescent agents, ensuring long shelf life.
5 Common Sourcing Pitfalls
B2B buyers often discover these issues only after the goods have arrived. We help you identify them before you pay.
1. The "Golden Sample" Trap
You receive perfect samples, but the bulk order has different dissolution times or color intensity due to poor batch control.
2. The "Ghost Certificate"
Suppliers provide compliance documents that look real but are expired, belong to another entity, or don't cover the specific dye ingredients used.
3. The Permanent Stain Risk
Cheap industrial dyes save money but stain lips and clothes permanently, leading to massive customer complaints and product recalls.
4. Delivery Chaos
Low MOQ promises often come with deprioritized production scheduling, leading to missed launch dates and stockouts.
5. The "Yes Man" Service
Sales reps who agree to every customization request without understanding technical feasibility, resulting in failed final products.
Tailored Solutions for Every Channel
Not all disclosing tablets are the same. We adjust the formula, flavor, and packaging to fit the specific needs of your end-user.
Dental Clinics
Chair-side patient education tools. Requires high contrast and instant results.
- Rapid Dissolve Formula
- Bulk Tub Packaging
Orthodontics
For braces wearers to check cleaning around brackets. High frequency usage.
- Purple Disclosure
- Starter Kits (10-pack)
Schools & Education
Mass oral health programs. Needs to be safe, fun, and cost-effective.
- Fruit Flavor Options
- Individually Wrapped
Retail Brands
Home care products. Packaging appeal and shelf life are critical.
- Blister Packs
- Custom Retail Boxes
Why Brands Are Moving to
Factory-Direct Sourcing
The era of buying generic tablets from trading companies is ending. Brands now require the control, compliance, and customization that only a direct manufacturing partner can provide.
Private Label Growth
Retailers demand unique packaging and branding, not generic white-label goods.
Stricter Regulations
EU MDR and FDA requirements demand direct access to technical files and factory audits.
Supply Stability
Direct communication eliminates the "broken telephone" effect during supply shortages.
ITS Dental Care Products
We are not just a vendor. We are the specialized manufacturing partner behind some of the world's leading oral care brands.
Dedicated Manufacturer
We focus exclusively on oral effervescent products. No distractions, just deep expertise in tablet compression.
R&D Collaborator
We don't just print your logo. We help you adjust formulas, test flavors, and optimize dissolution times.
Long-Term Partner
We build relationships measured in years, offering stable pricing, consistent quality, and growth support.
How We Improve the Product,
Not Just Copy It
Formula Tuning
We adjust effervescent ratios to ensure rapid breakdown without compromising structural integrity during shipping.
Color Calibration
We test dye concentrations to achieve the perfect balance: visible on plaque, but easy to rinse off tissues.
Batch Traceability
Every lot is tracked from raw material to final carton, ensuring total accountability for your brand.
Regulatory Adaptation
We proactively adjust labeling and documentation to meet evolving standards in your target export market.
Planning Your Project?
5 questions to answer before you finalize your sourcing strategy:
The Industry is Evolving.
Choose a Partner Who Leads It.
Plaque disclosing tablets are moving from "accessory" to "standard tool."
The real value comes from working with people who understand this shift long-term.
